
A Detailed Look At The 5 Factors Of Low Voltage
The following blog post is taken directly from a Senergy Facebook Livestream with Dr. Jerry Tennant. Watch the video below! The 5 Factors of Low
Functional Medicine Services in Dallas / Fort Worth Area
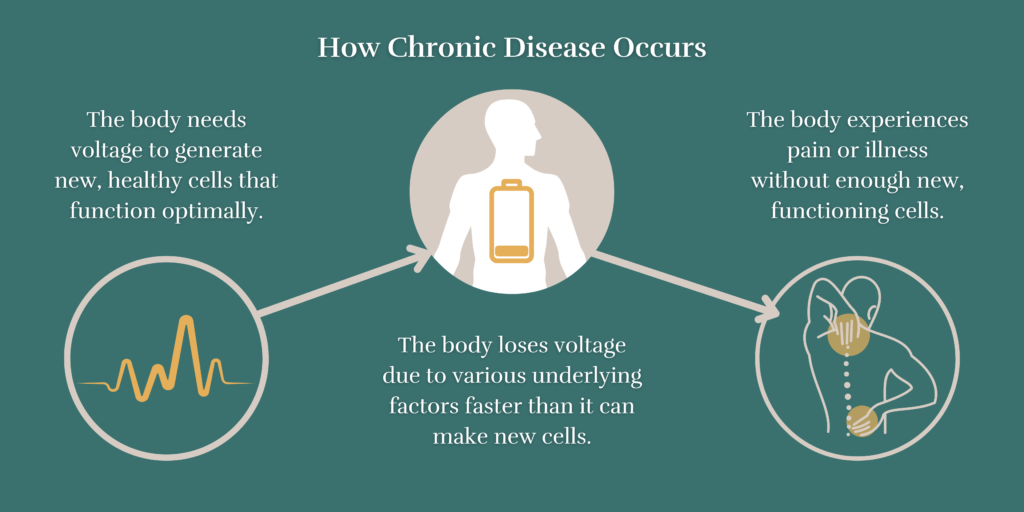
Low voltage in the body leads to constant sickness and slow recovery times. Many people cannot heal their chronic illnesses because they never investigate the root cause of low voltage in their body.
If you suffer from any of these conditions, you need to get your voltage tested today.

Your health is our first priority. You won’t have to take second seat to insurance companies or hospital bureaucracy. We don’t push pharmaceuticals for profit. Our treatments focus on you and you alone.

We don’t take an either/or approach. We embrace all forms of legitimate medicine to create your treatment plan. From nutrition supplements to hormone therapy, you’ll have access to a range of science-based options.

We stay with you on every step of the journey and leave no stone unturned. There is no one-size-fits-all when it comes to health. Your care plan will be unique to you and your health history/concerns.

Do you have enough voltage in your system for your body to heal? At the Tennant Institute, we determine this by doing baseline testing of your overall voltage, the performance of each polarity, and a test to measure how your body is processing heavy metals. This gives the doctors information on what physical challenges your body is facing when it doesn’t have the underlying power to operate and regenerate healthy cells.

Tennant Microcurrent technology is used to give your body what it needs to generate new, healthy cells that heal. This is achieved by correcting your polarities with a Tennant BioTransducer®, and recharging with the FDA-accepted Tennant BioModulator®. This device is used with simple electrodes that effectively deliver healing frequencies into your body.

Once the doctors have determined your overall voltage measurements, they then begin the investigation of why your voltage dropped. There are five areas we focus on that consistently will cause a drop in body voltage: dental infections, thyroid hormone imbalances, stuck emotions, scars, and toxins. During your time at the clinic, you will meet with our practitioners to investigate and resolve these issues that often serve as the root cause of disease.
We take each individual’s health concerns and their medical history into consideration when recommending a care plan. Click the button below to learn more.






A world leader in integrative and functional medicine.
Genius, scholar, inventor, humanitarian, innovator, healer, teacher, entrepreneur, historian — Dr. Jerry Tennant has led a remarkable life dedicated to healing and innovation. Over the course of his career, he has changed the paradigm of western medicine.
Today, people travel from around the world to consult with the Tennant Institute for Integrative Medicine. Dr. Tennant also teaches and lectures worldwide for Senergy, the exclusive distributor of his patented Tennant BioModulator® PLUS and PRO. The countless thousands of success stories and testimonials of patients’ recovery and pain reduction are phenomenal.
The “why” behind what we do
Dr. Tennant’s Daily Protocol will reset your body’s natural polarities and recharge your cells with the voltage they need to heal.
This is achieved with Dr. Tennant’s Microcurrent Frequency devices, the Tennant BioModulator® and Tennant BioTransducer®.
Watch this video to learn how the devices work and just how easy it is to restore voltage to your cells!
Read Our Blog

The following blog post is taken directly from a Senergy Facebook Livestream with Dr. Jerry Tennant. Watch the video below! The 5 Factors of Low

What Supplements Are Good For Lupus? If you or someone you know has lupus, it’s not surprising that the patient would be on a quest

How To Strengthen Your Immune System Taken from a Senergy Friday Livestream Interview with Tennant Products director and product developer Scott Jessen. To watch the
Schedule a free consultation by clicking the button below!
***We do not serve as your primary care physician. We are not an emergency facility. We cannot assist those in a hospital. For emergencies, contact your primary care physician or call 911.
***We DO NOT participate with any insurance including Medicare or Medicaid